Regulatory Compliance
Is certification of document copies a regulatory requirement under EMA or MHRA guidelines?
Certification is not explicitly mandated in EMA or MHRA regulations. However, EMA’s Reflection Paper on TMF and guidance from national regulators (e.g., MHRA, BfArM, ANSM) emphasize the importance of traceable document destruction and complete audit trails. As a result, certified copies and certificates of destruction are considered best practices to support inspection readiness.
In the Certified Copy requirement, does “EU” refer to the European Union, and is the concept specific to EU regulations?
“EU” refers to European regulatory best practices outlined by agencies such as EMA, MHRA, BfArM, and ANSM. The concept is not exclusive to the European Union. Certified Copies are recognized in both EU and FDA frameworks as part of inspection readiness. The earlier reference to “ET” was a typographical error and has been corrected to “EU.”
Is “EU” the correct term in the requirement, and what does it refer to?
Yes. “EU” is the correct term and refers to European regulatory best practices related to certified copies and inspection readiness.
What is HITRUST?
HITRUST (Health Information Trust Alliance) is a framework designed to help organizations manage data protection, information risk, and regulatory compliance, particularly in healthcare. Originally developed to support HIPAA compliance, HITRUST has since expanded to be used across multiple industries.
What is ISO 27001?
ISO 27001 is an international standard for managing information security. It provides a framework for establishing, implementing, maintaining, and continually improving an Information Security Management System (ISMS) across organizations of any size or industry.
How does HITRUST compare to ISO 27001?
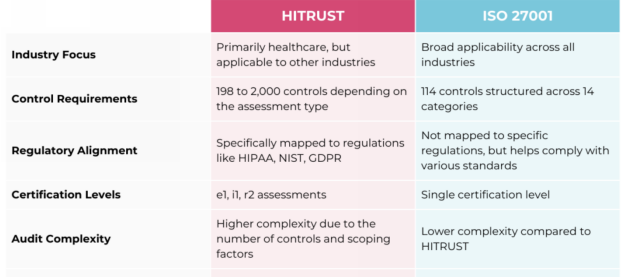
Do HITRUST and ISO 27001 differ from a compliance perspective?
Yes. ISO 27001 focuses on general information security management, while HITRUST includes additional controls specifically designed for handling sensitive data such as Protected Health Information (PHI). HITRUST is generally considered more comprehensive due to its inclusion of HIPAA and other regulatory requirements.
Can HITRUST be considered more comprehensive than ISO 27001?
Yes. HITRUST builds upon ISO 27001 by incorporating industry-specific regulatory requirements such as HIPAA and GDPR, making it more suitable for organizations handling sensitive data.
Why should HITRUST be prioritized over ISO 27001 when discussing compliance?
HITRUST offers broader regulatory coverage and aligns more closely with industries managing PHI and other sensitive data. Prioritizing HITRUST demonstrates stronger compliance maturity and alignment with evolving regulatory expectations.
How can compliance certificates such as HITRUST or ISO 27001 be securely shared?
A Virtual Data Room (VDR) can be used to securely share compliance certificates and NDAs. VDRs provide access controls, audit trails, and permission management to ensure secure and traceable document sharing.
What certifications do Trial Interactive and its hosting providers hold?
Trial Interactive is hosted on AWS, which operates SSAE 16 SOC 2–certified data centers. The application is managed according to Trial Interactive policies, including SDLC and Change Management procedures.
Are there additional requirements for customers to remain compliant with the EU 95/46 data privacy directive?
No. Trial Interactive operates as a Data Processor, while customers remain Data Controllers. No additional requirements are imposed beyond those assigned to data controllers.
Is Trial Interactive compatible with GDPR?
TransPerfect continuously evaluates GDPR requirements. At present, no significant changes are anticipated. Customers will be informed of any identified impacts following assessment.
Is Trial Interactive compliant with 21 CFR Part 11 for electronic signatures?
Yes. Trial Interactive provides the necessary system controls to support compliance with 21 CFR Part 11 for electronic records and electronic signatures.
Does Trial Interactive support agency requirements for electronic signature certifications?
Yes. Trial Interactive provides reporting capabilities that allow organizations to submit a single certification covering all applicable users, as permitted under 21 CFR 11.100.
What is the Trial Interactive Privacy Policy?
Trial Interactive follows TransPerfect’s privacy policy, which outlines how customer data is processed and protected. The policy is accessible via a link within the application.
How is Trial Interactive user data handled?
Trial Interactive uses a multi-tenant SaaS architecture that ensures complete logical separation of each customer’s data while providing scalability and security.
Are uploaded documents securely stored?
Yes. All documents are virus-scanned and encrypted both at rest and in transit.
What is Trial Interactive’s documentation and SOP strategy?
Trial Interactive maintains controlled SOPs under QA governance. Employees receive mandatory training, and customers may review documentation in supervised audit settings.
Does Trial Interactive accommodate customer audits?
Yes. Customers may conduct formal audits at corporate offices, while hosting audits are supported through standardized certifications such as SSAE 16 SOC 2 reports.


 Linkedin
Linkedin
 X
X

