Amendments
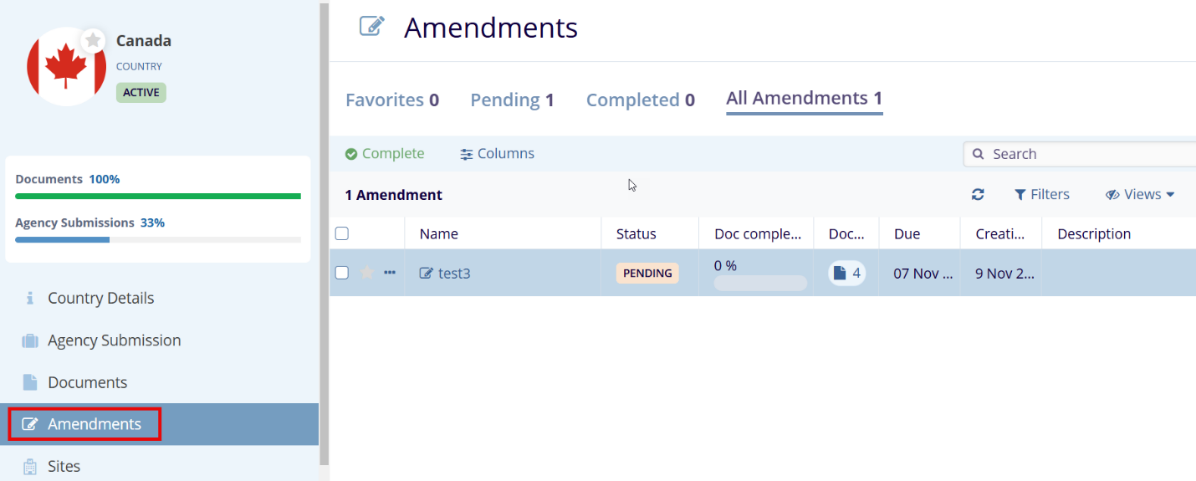
Once a clinical trial begins, protocol amendments often require sites to submit additional essential documents. These documents can only be specified through Amendments, which can be created for Investigative Sites, Country Amendments, and IRB/EC.


 Linkedin
Linkedin
 X
X

