Edit Studies
Explore Job Aids and Videos for more help:
Job Aids
Videos
To edit studies, follow the steps below:
- Click on the check box next to the name of the study.
- Click on the Edit button from the top main menu.
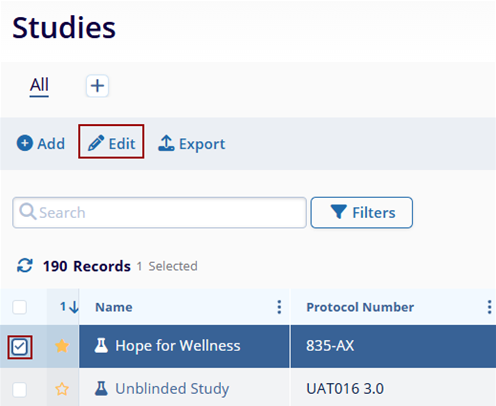
- Make any necessary changes to the Study Details by expanding the following sections.
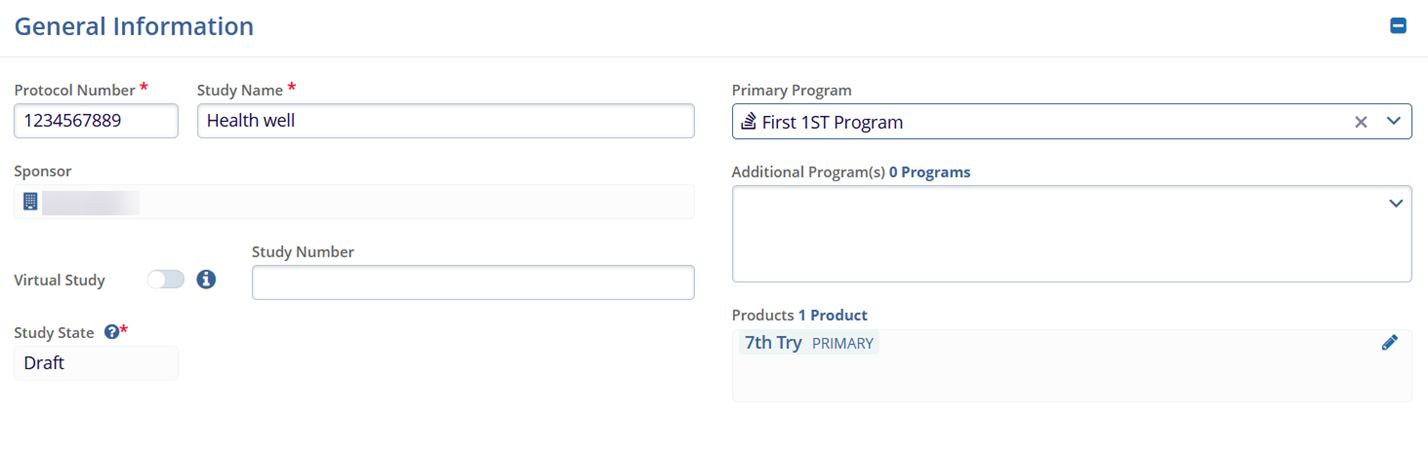
- General Information:
- Protocol Number
- Study Name
- Sponsor
- Primary Program
- Virtual Study
- Study Number
- Additional Program(s)
- Study State
- Products
-
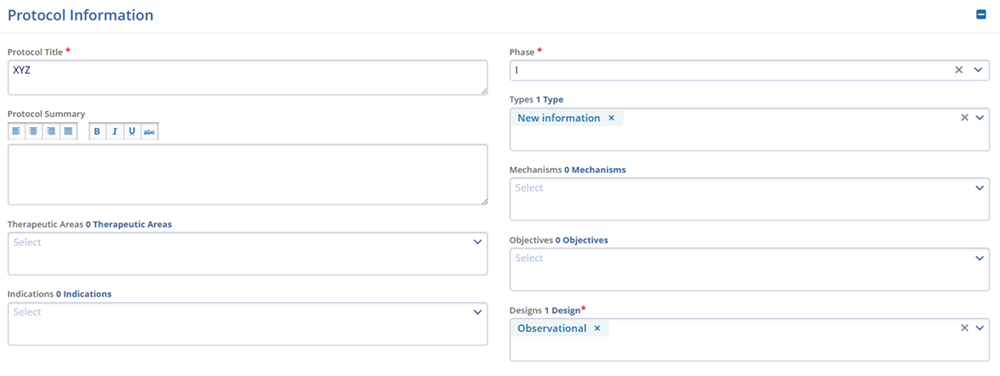
Protocol Information
- Protocol Title*
- Protocol Summary
- Indications
- Phase*
- Types
- Mechanisms
- Objectives
- Designs*
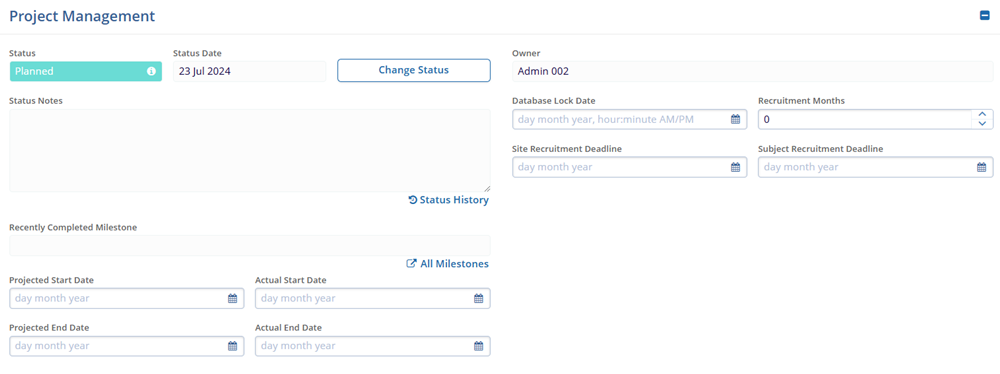
- Project Management
- Status
- Status Date
- Status Notes
- Recently Completed Milestone
- Projected Start Date
- Projected End Date
- Actual End Date
- Owner
- Database Lock Date
- Recruitment Months
- Site Recruitment Deadline
- Subject Recruitment Deadline
- Subject and Recruitment
- # Planned Trial Sites
- 1st Site Activated
- # Planned Subjects Entered Trial
- # Planned Subjects Entered Treatment
- # Planned Subjects Completed Treatment
- Actual Screened
- Actual Enrolled
- Actual Screen Failed
- Actual Completed.
Didn’t find what you need?
Our dedicated Project Management and Client Services team will be available to meet your needs
24 hours a day, 7 days a week.
Toll Free:
(888) 391-5111
help@trialinteractive.com
© 2025 Trial Interactive. All Rights Reserved


 Linkedin
Linkedin
 X
X

