eTMF module
The eTMF module serves as a central access point for Clinical Trial Documents, eTMF Sites, Contacts, eTMF Completeness, and CRA Reconciliation Reports for clinical trial activities. It also provides access to IRB (Institutional Review Board) Integration and Potential Sites.
Users can access this module from the 'Navigation Grid' in the upper-left corner of the screen, where the eTMF module is listed below.

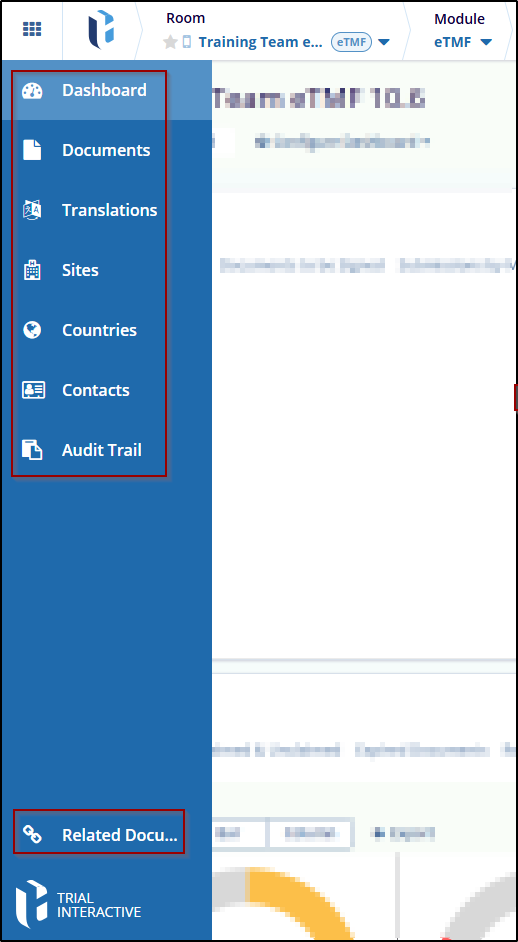
The eTMF module includes:
- Dashboard
- Documents
- Translations
- Sites
- Countries
- Contacts
- Audit Trial
- Related Documents Settings
Refer to the screenshot below:
Note: The Toolbar options are available per the room settings and user access.
Navigate to the Toolbar on the left panel of the screen and click on the Dashboard section.


 Linkedin
Linkedin
 X
X

