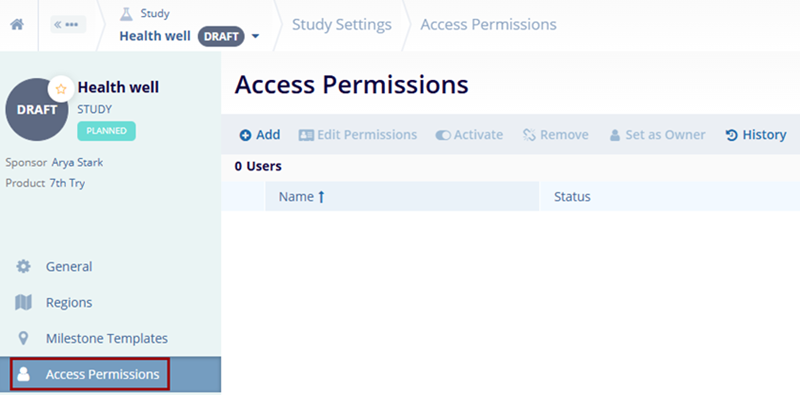
Access Permissions
Explore Videos for more help:
Videos
To manage the access permissions from the Study Settings, follow the steps below:
- Navigate to the Study Settings by following the steps detailed in the Study Settings section.
- Click on the Access Permissions link from the left-hand navigation pane.
- Refer to the Managing Study Access
Permissions section to understand the standard steps.
Didn’t find what you need?
Our dedicated Project Management and Client Services team will be available to meet your needs
24 hours a day, 7 days a week.
Toll Free:
(888) 391-5111
help@trialinteractive.com
© 2025 Trial Interactive. All Rights Reserved


 Linkedin
Linkedin
 X
X

