Reject Site Visit Report
When CRAs create a site visit report and submit it for review, the designated reviewer (usually the Clinical Study Manager) has the option to reject the report.
To reject a site visit report, follow the steps below:
- As a CSM, navigate to the Site Visit General Information screen by following the steps detailed in the Site Visit Navigation section.
- Navigate to the Visit Report section, click the View Report link to open the report in TI Viewer.
- Click on the Reject button.

- Specify a rejection reason and click Reject.
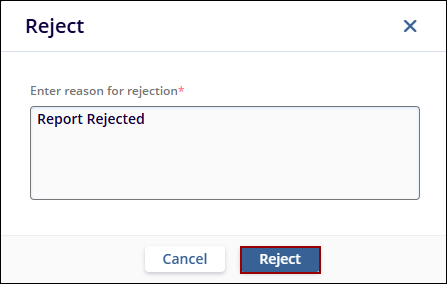
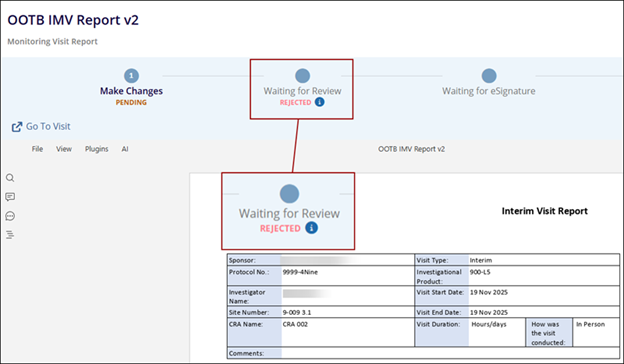
- The report status is displayed as Rejected.
Didn’t find what you need?
Our dedicated Project Management and Client Services team will be available to meet your needs
24 hours a day, 7 days a week.
Toll Free:
(888) 391-5111
help@trialinteractive.com
© 2025 Trial Interactive. All Rights Reserved


 Linkedin
Linkedin
 X
X

