Documents Types Management
This section describes the settings associated with the Document Types
In the conduct of a clinical trial, scores if not hundreds of different kinds of documents need to be collected, categorized, and filed – some general documents, some documents that are specific to the countries in which studies are being conducted, and some documents that are specific to the investigative sites involved in the study.
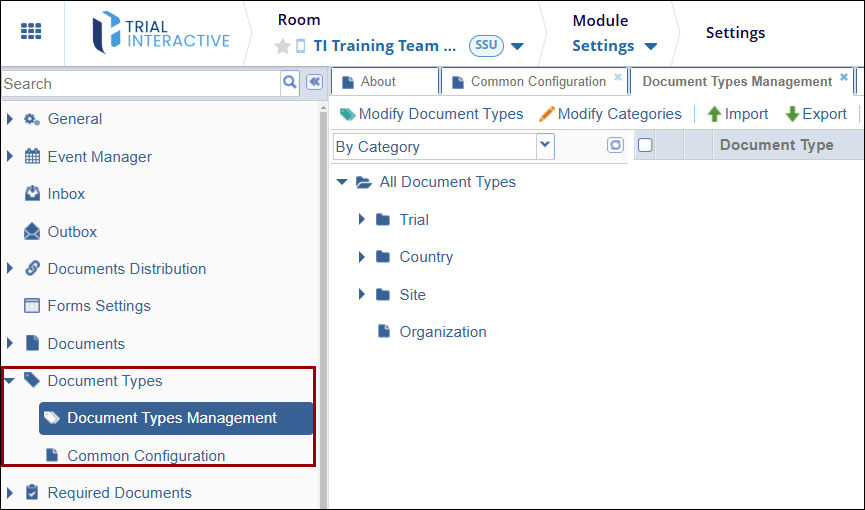
To access the Document Type Settings, follow the steps below.
- Navigate to the Settings module.
- Expand the Document Types setting dropdown.
- Select the Document Types Management or Common Configuration option.


 Linkedin
Linkedin
 X
X

